Vial
Object number2021.141.1
Manufacturer
Quintiles, Inc.
Datec. 1988
OriginKansas City, MO
MediumGlass; Plastic; Paper; Ink; Adhesive
Credit LineGift of Raman Venkataramanan
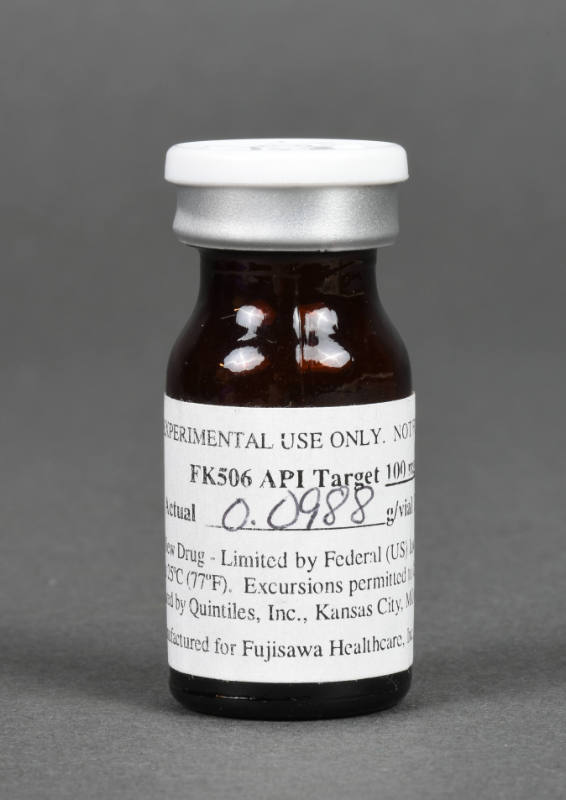
DescriptionMedical vial. Cylindrical brown transparent glass bottle with white and silver plastic lid. Bottle has two seams. White paper label adhered to the side of the bottle. Bottle appears to be empty.DimensionsHeight x Diameter: 2.063 × 0.875 in. (5.2 × 2.2 cm)InscriptionsLid has raised text on top "FLIP / OFF".
MarksWhite paper label adhered to the side of the bottle has printed and handwritten text "FOR EXPERIMENTAL USE ONLY. NOT FOR HUMAN USE. / FK506 API Target 100 mg/vial / Actual 0.0988 g/vial Lot #520 / Caution: New Drug - Limited by Federal (US) to investigational use. / ... / Prepared by Quintiles, Inc., Kansas City, MO 64137... / [logo] Manufactured for Fujisawa Healthcare, Inc., Deerfield, IL 60015".
Underside has raised number at center "20".
Historical NotesVial of Tacrolimus (FK506) used in early testing of the chemical. Tacrolimus, also known as FK506, is an immunosuppressive drug that is given orally or intravenously to people who have had a kidney, liver, heart, or other organ transplant. On March 1, 1989, it was administered via intravenous injection to the first person, a liver transplant patient.
Related institution
Fujisawa Healthcare, Inc.
Related person
Raman Venkataramanan
On View
Not on view1980-1989
May Drug Company
Natrona Bottling Company
American Safety Table Company